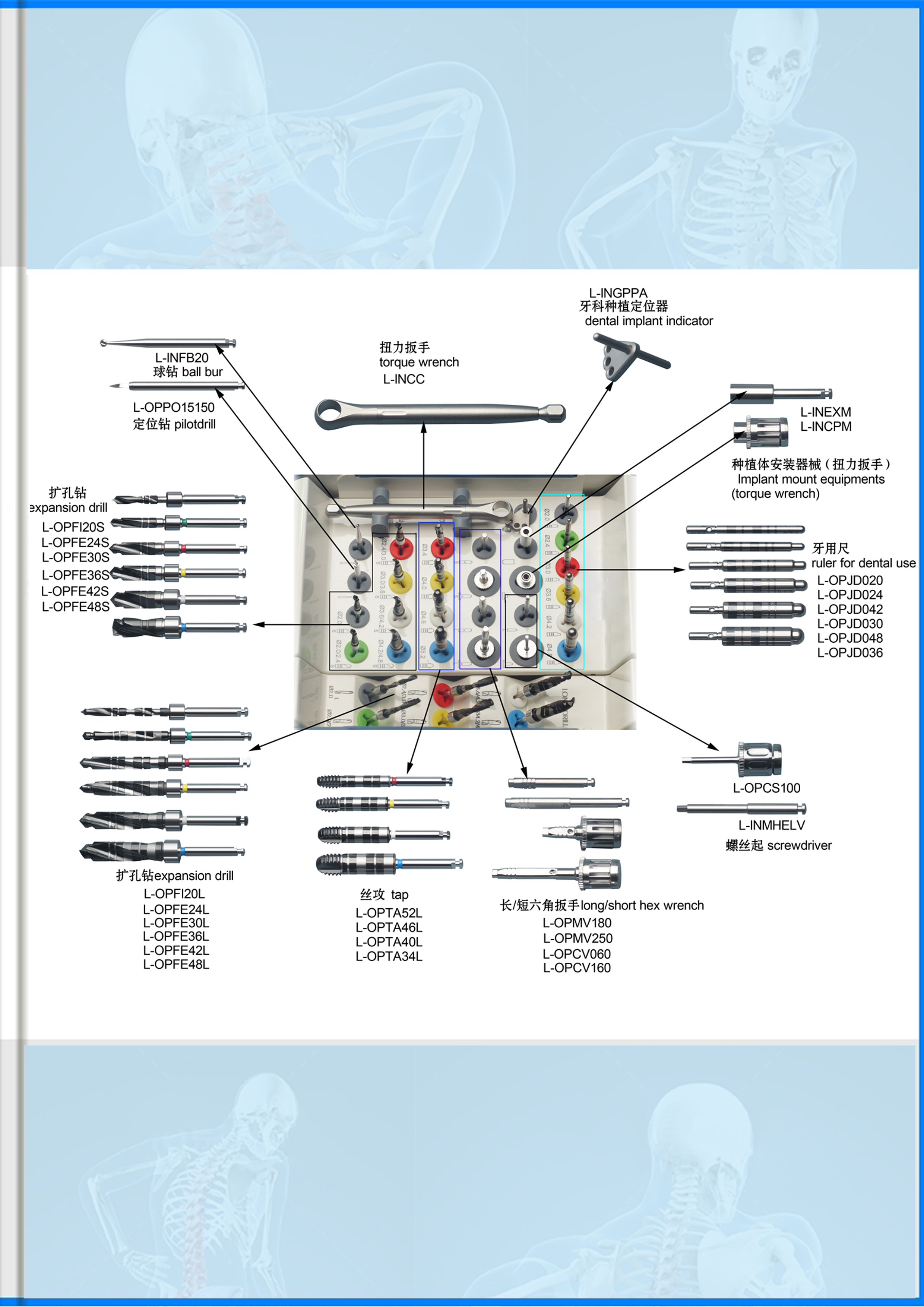
Medical Screw Tap
Application domain: for Implant
Material: Super-high rust-resistant high-hardness stainless steel,
ceramics, general rust-resistant stainless steel
、Medical Screw Tap P152
Characteristics
Application domain: for Implant
Material: Super-high rust-resistant high-hardness stainless steel,
ceramics, general rust-resistant stainless steel
Products Description
LZQ is an OEM factory for all kinds of dental implant tap, such as: Abutment/Transfer P25,Parallel Pin P37, Wrench P53, Screwdriver P79, Tap P152, Bone Fetcher P165,Lance Drill P172, etc. We can also produce a whole kit of tools for customers’ surgical cases.
We use the material of stainless steel.
We are capable to produce any taps according to drawings or samples provided by customer with a favorable cost-performance.
Material:
- Super-high anti-rust high wear resistance stainless steel series
(AA)(HRC54°±2°section)(regularly stock)
2. General anti-rust high wear resistance stainless steel series
(A)(HRC45°~64°section series)
(Ultra-high rust-proof stainless steel AA)
(hardened HRC54 ° ± 2 ° Section)

Ultra-high anti-rust & high wear-resistant , high hardness, high impact, high toughness stainless steel, with excellent anti-rust capability, corrosion resistance, wear resistance (high hardness) performance and ideal impact resistance.. Its excellent corrosion resistance is comparable to TYPE 304 Stainless steel; and its rust resistance is closer to alloy for surgical implant. Unmarked, with 5% Neutral salt spray test (ASTM B117), it doesn't rust after 1000 hours. Wide range of applications, quite good and stable quality, which can be used to produce extremely complex, high-precision shank shapes and profile ultra-sharp edge structures.
Applied for high-end medical standard:
AMS 5936 . MMPDS-01 . ASTM A693
ASTM A564 . ASTM F899-12
The Vitro (cytotoxicity) test verifies that the material does not have any potential cytotoxicity and therefore can safely contact human tissue, body fluids or blood, and meets all relevant allergy and skin irritation test standards.
When you make inquiry, referencing to the page footer, please provide samples with best work performance, and specify the detailed requirements, material types, dimensional tolerances, drawings of finished and semi-finished products, equipment used or equipment value (to judge its rigidity and runout, etc), monthly consumed qty, type of original samples and its inadequacies, the workpiece material to be processed by sample and its hardness HRC? ...